The Natural Modes of Nitrogen Fixation Are
Nitrogen is also the most abundant element in the atmosphere 78. It is done by few forms of bacteria which may be free living like A z o t o b a c t e r or symbiotic bacteria like R h i z o b i u m which can convert insert nitrogen molecule into forms like nitrates and nitrites which can be taken up by plants and used to make the required molecules proteins.
Much like in plants proteins can help control metabolic activity in humans.
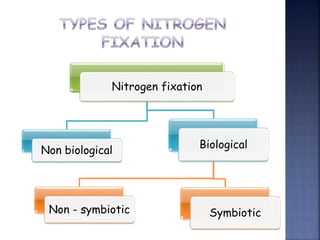
. In an effort to improve our understanding of how microbial nitrogen fixation works two research teams. 1 Physical Nitrogen Fixation and 2 Biological Nitrogen Fixation. Thus we can classify nitrogen fixation in following two types.
Nitrogen fixation occurs in a varied metabolic context in both aerobic and anaerobic environments. Only 10 of natural nitrogen fixation takes place by physicochemical means whereas 90 is carried out by biological means. Because the nitrogenase proteins are denatured by exposure to oxygen O 2 they can only operate in an anaerobic environment.
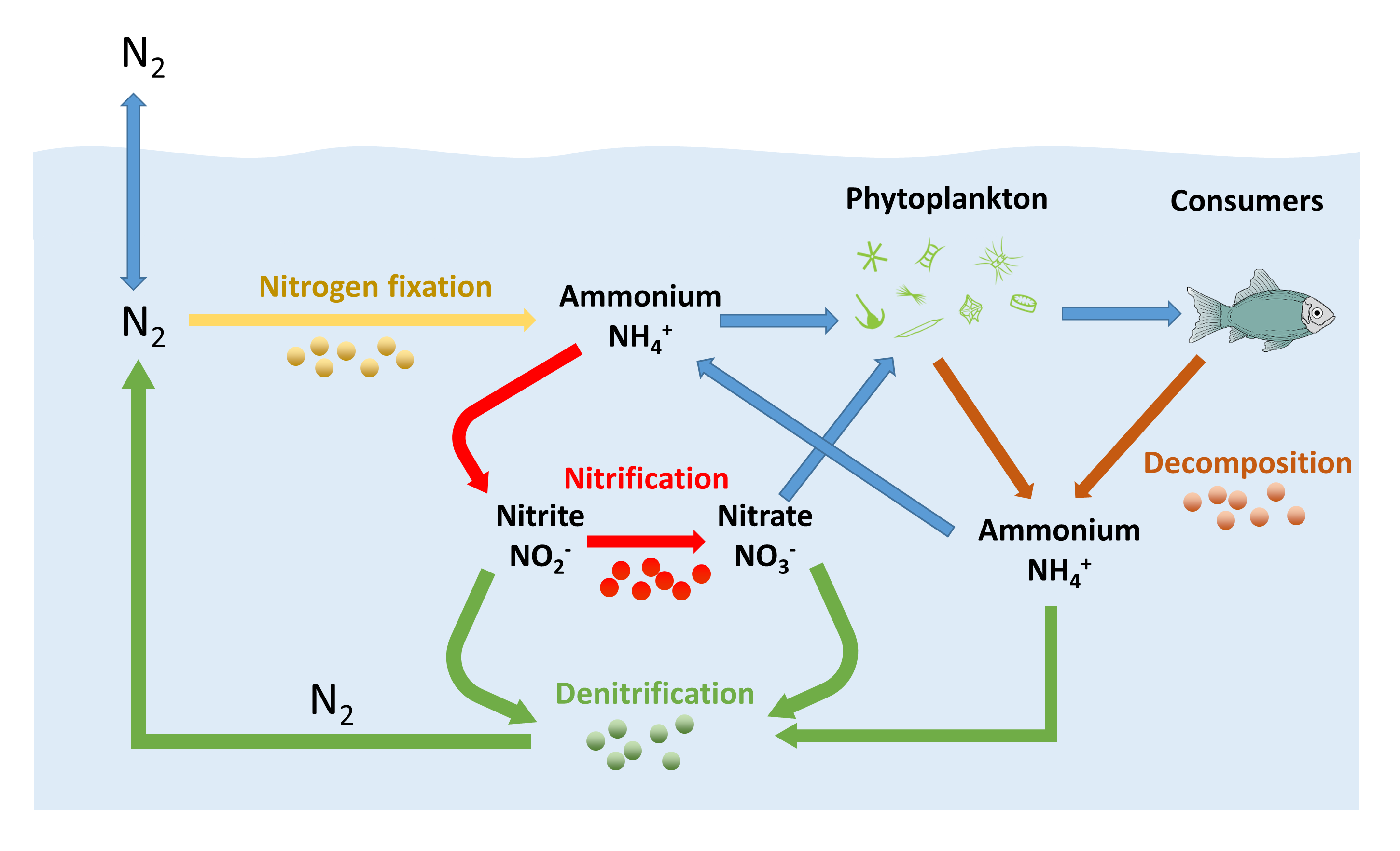
The nitrogen cycle describes the path of the element nitrogen through nature. The two types of nitrogen fixation are. Biological nitrogen fixation is accomplished through the action of an enzyme known as nitrogenase.
Bacteria Microorganisms that fix nitrogen are known collectively as diazotrophs. Nitrogen fixation is a chemical process by which molecular nitrogen N. Nitrogen fixation by nature lightning strikes fires and UV radiation convert nitrogen gas into nitrates and nitrites in the soil nitrogen fixation by man industrial processes are used to create nitrogen rich fertilizers which farmers put on their fields.
These compounds are formed by the process known as nitrogen fixation which can only be carried out in nature with the help of microorganisms. These fertilizers contain nitrates nitrites and ammonia nitrogen fixation by bacteria. 2 Fe protein transfers two electrons to the FeMO protein and hydrolyzes 4ATP.
Another process that helps in. Abiotic natural inducers are lightning and UV rays. There are two key methods of natural nitrogen fixation.
However gaseous nitrogen must be fixed into another form so that it can be used by living organisms. Alternatively N can be fixed with electrical equipment or industrially. Nitrogen fixation by bacteria is an example of the symbiotic relationship between Rhizobium and leguminous plants.
Enzymes are also found in humans and help regulate the rate of. Some other methods of nitrogen fixation are Habers process atmospheric nitrogen combines with hydrogen under the influence of Fe while Mo acts as a promoter. Apart from carbon hydrogen and oxygen nitrogen is the most prevalent essential macro-element in living organisms.
In agriculture fertilizers are often deployed to supplement nitrogen levels in poor soil. Nitrogen fixation is the natural biological process by which nitrogen N2 in the atmosphere is converted into ammonia NH3. Natural nitrogen fixation is performed by a number of microorganisms called diazothrops.
37 Natural modes of nitrogen fixation are A various industrial processes of making fertilizers. Ii Biological nitrogen fixation. 2 with a strong triple covalent bond in the air is converted into ammonia NH.
While bacteria fix nitrogen in the soil plants provide them food. 1 Fe proteins receives 2 electrons from reduced ferrodoxin and binds 4 ATP. Lightning Lightning provides energy to react water H 2 O and nitrogen gas N 2 to form nitrates NO 3 and ammonia NH 3.
Nitrogen Fixation by Lightning. Nitrogen can be fixed by lightning that converts nitrogen and oxygen gas found in the atmosphere into nitrogen oxides. More than 90 percent of all nitrogen fixation is effected by them.
The conversion of atmospheric nitrogen into ammonia and organic derivatives by natural means especially such conversion by microorganisms in the soil into a form that can be assimilated by plants Nitrogen fixation also refers to other biological conversions of nitrogen such as its conversion to nitrogen dioxide. Non-biological or Physical nitrogen fixation. Nitrogen fixation in nature Nitrogen is fixed or combined in nature as nitric oxide by lightning and ultraviolet rays but more significant amounts of nitrogen are fixed as ammonia nitrites and nitrates by soil microorganisms.
Another method is the Birkeland Eyde process. B fossil fuel combustion. Yale University scientists may have cracked a part of the chemical code for one of the most basic yet mysterious processes in the natural world -- natures ability to transform nitrogen from the.
Nitrogen plays essential roles for plants and animals. Humanity depends on fixed nitrogen to fertilise croplands and helping nature in relation to nitrogen availability is a key aspect of world food security. FeMo protein binds 2H which are reduced by electrons to H2.
Nitrogen fixation is carried by physicochemical and biological means. Atmospheric nitrogen is molecular dinitrogen a relatively nonreactive molecule that is. Nitrogen fixation the conversion of inert nitrogen to ammonium ammonia nitrate or nitrogen oxides is a process which occurs naturally but can also occur through anthropogenic processes.
D denitrification and various industrial processes. About 90 of natural N fixation on our planet is biotic and occurs thanks to soil microorganisms. Yet nitrogenase is irreversibly inactivated by oxygen in vitro although reversible oxidation has been observed in vivo Zehr et al.
Rain and snow carry these compounds to the surface where plants use them. Chemically N fixation is splitting the triple bond in N2 and reducing it to ammonia NH3 or ammonium NH4. Nitrogen is essential for lifeit is found in amino acids proteins and genetic material.
Nitrogenase consists of two distinct proteins which contain molybdenum iron and sulfur. N2 displaces the H2 on the FeMO protein. What are the steps of the nitrogenase cycle.
C lightning and bacterial fixation in soils. 3 or related nitrogenous compounds typically in soil or aquatic systems but also in industry.
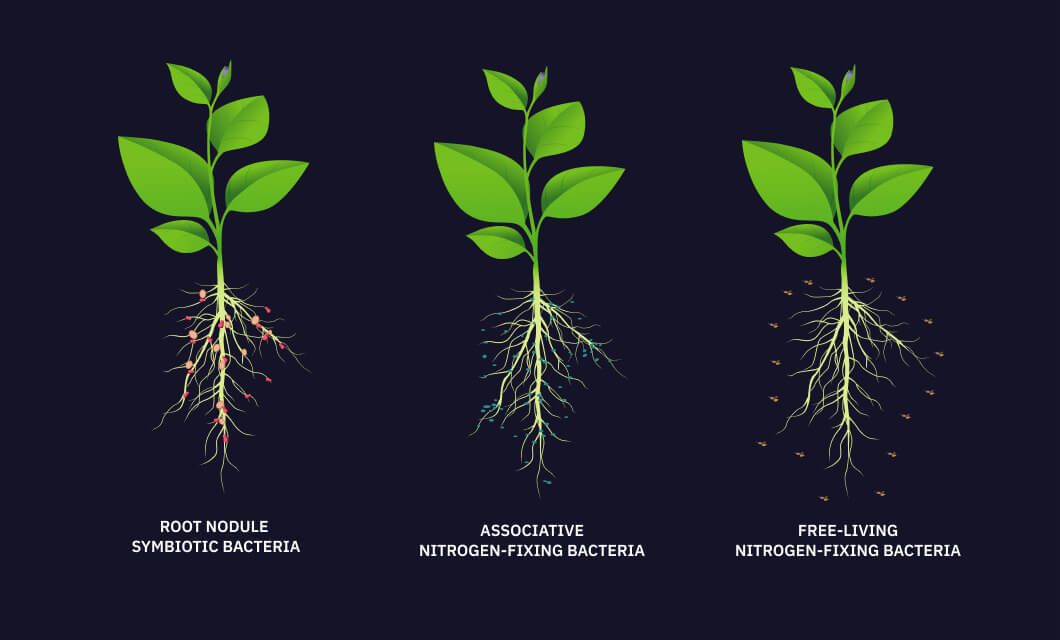
Nitrogen Fixation N Fixing Plants Bacteria Their Importance

Nitrogen Fixation Definition Process Examples Types Facts Britannica

Nitrogen Fixation Definition Process Examples Types Facts Britannica
Comments
Post a Comment